Introducing our NEW all-in-one dressing
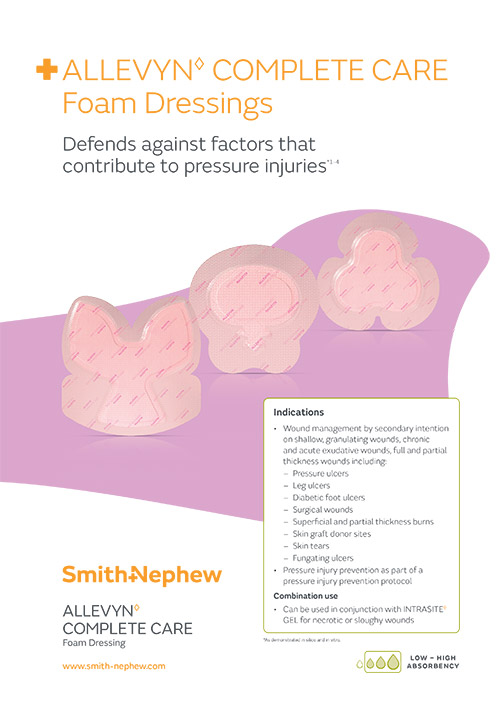
ALLEVYN◊ COMPLETE CARE Foam Dressing
ALLEVYN COMPLETE CARE Dressing mode of action
Chronic wounds can cost you
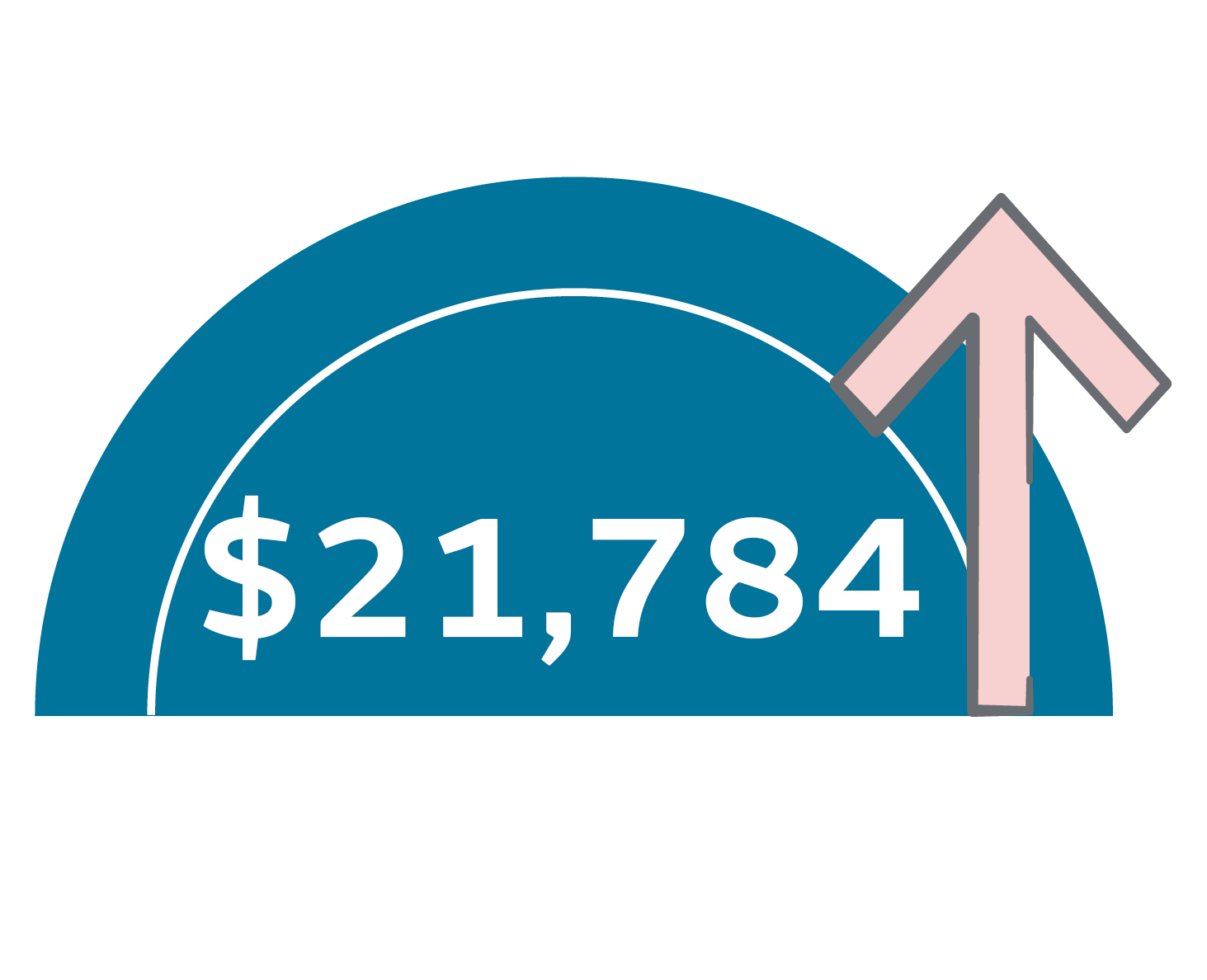
Patients with HAPIs in the US: Cost an additional $21,7841 and spend an extra 9.5 days in hospital²
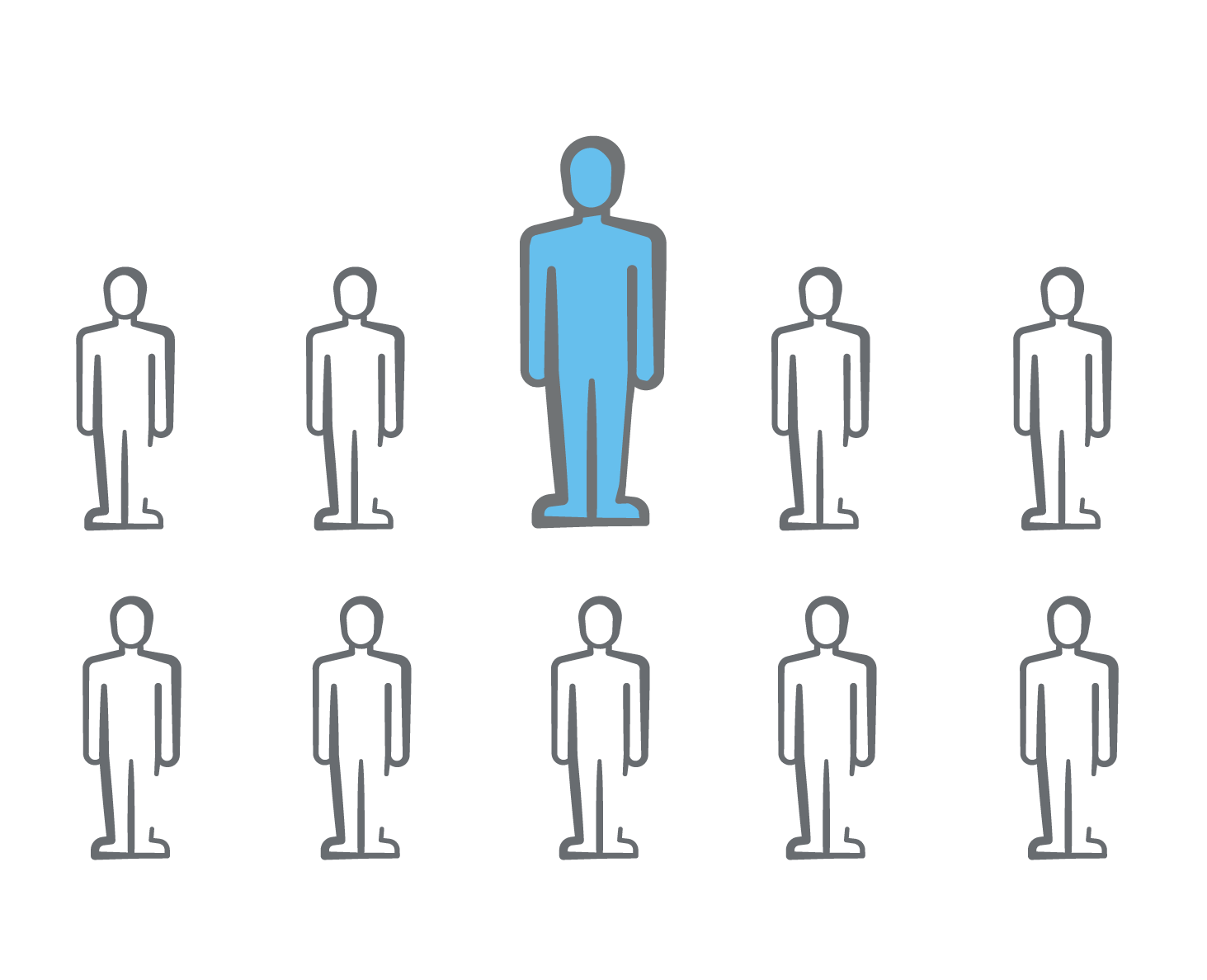
1 out of 10 adults are affected by hospital-acquired pressure injuries (HAPIs)2
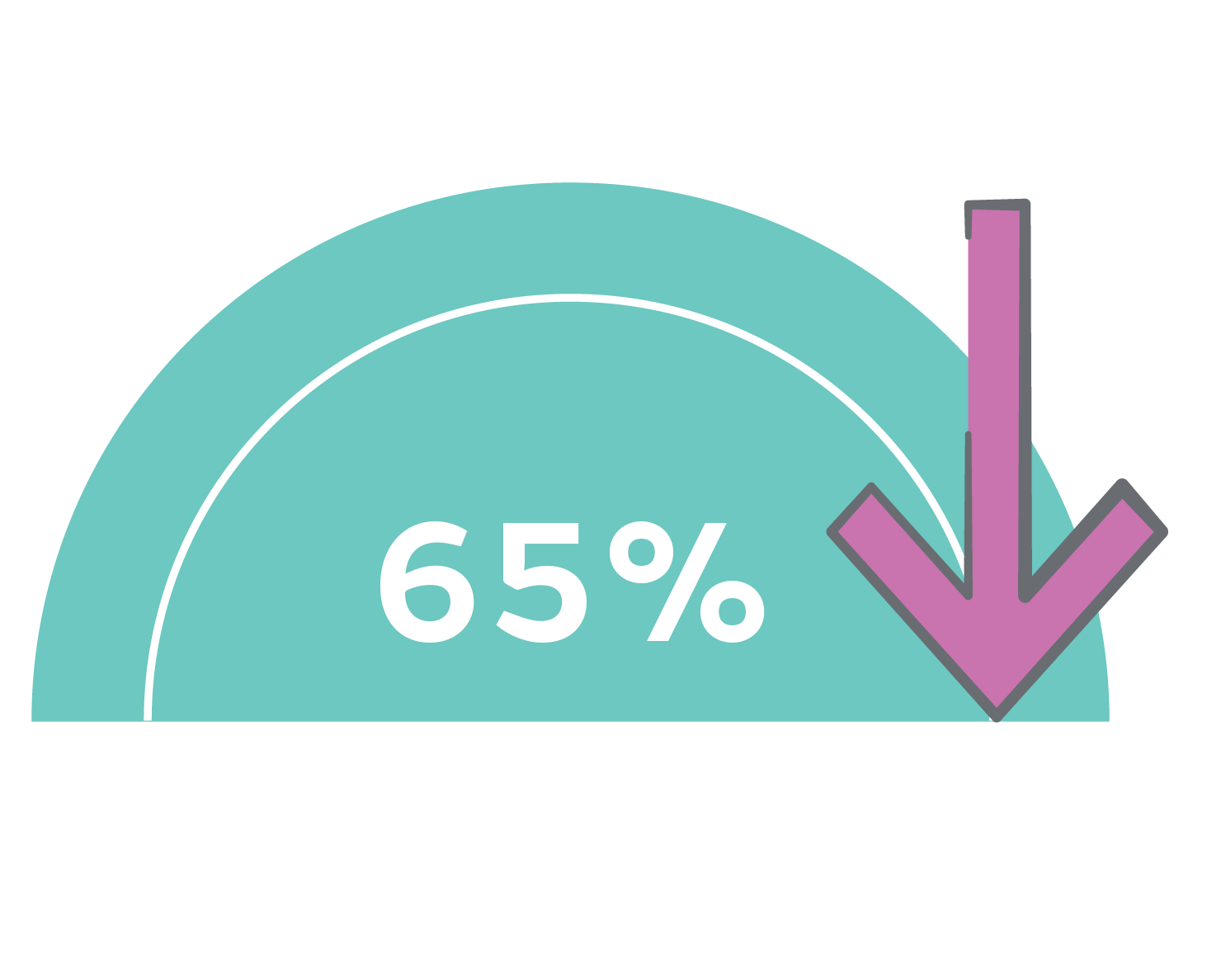
ALLEVYN 5 Layer Dressings reduce pressure injuries and associated costs by more than 65% with ShearDEFENSE◊ Technology*3-5
Reasons to change to ALLEVYN COMPLETE CARE Foam Dressings


Multi-Layer Design
*As demonstrated in vitro
Various fits
*as part of a prevention bundle Refer to IFU – MDRPIP is covered under PIP.
Pressure injury prevention
Visual indicator
Longer lasting
ALLEVYN 5 Layer Dressings can be worn nearly 30% longer than other foam dressings*16
*versus previous dressings (includes: other foam dressings, gelling fibers, protease-modulating dressing, contact layers, etc.); Mean difference of -0.85 change per week (2.91 vs. 2.06); 95% CI: -1.62 to -0.09; p=0.029; n=307
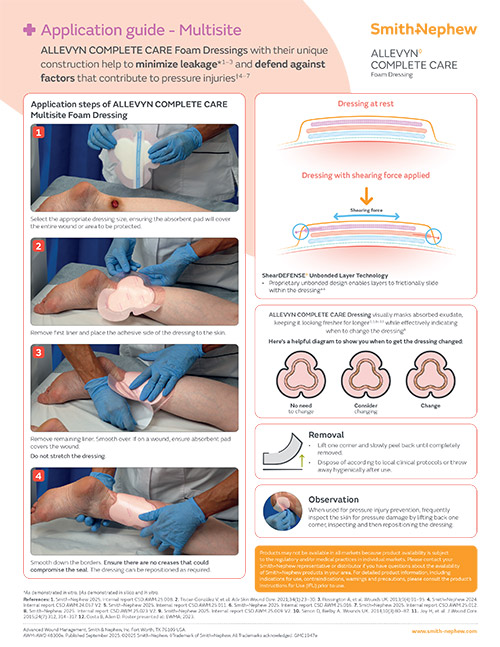
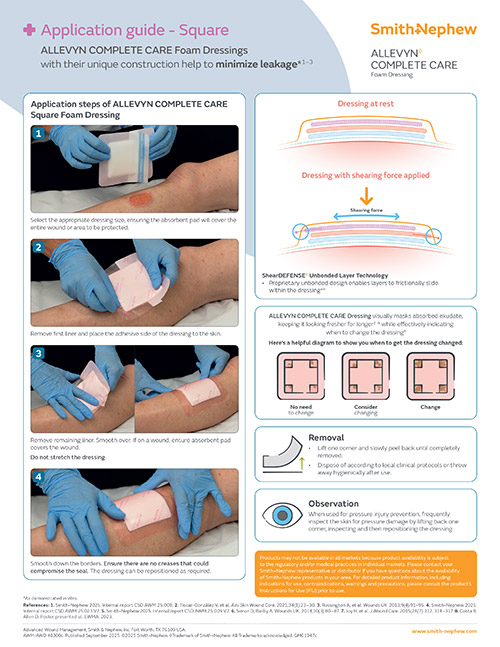
ShearDEFENSE Technology
ALLEVYN COMPLETE CARE Dressing is a unique dressing that has been proven to deliver frictional energy absorber effectiveness within the dressing due to its truly independent layers.**19
**As demonstrated in vitro
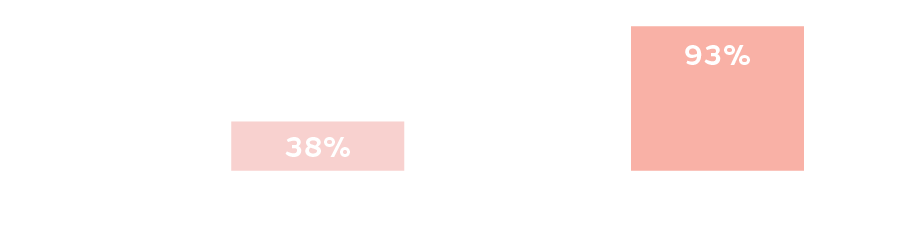
New evidence
ALLEVYN COMPLETE CARE Foam Dressings absorb up to 93% of mechanical energy into its internal layers20
How it works
Discover how the 5-layer design of ALLEVYN COMPLETE CARE Foam Dressings helps with the management of acute and chronic wounds.10,11
- Breathable bacterial barrier
- EXUMASK◊ visual indicator
- EXULOCK◊ lock-in technology
- Absorbent hydrocellular foam
- ComfortSTAY◊ gentle silicone adhesive
Patient support material
Connect with us to request a demo
Share your email address for more information about how ALLEVYN Dressings can help your facility reduce costs and patient recovery times.

Related products
*n=37; dressing retention was 1.92x longer.
**In a pragmatic, randomized, controlled, superiority trial. p=0.001; n=359. Estimated cost savings vs standard preventive care alone; n=359.
***Compared to previous dressings. Ambispective (retrospective and prospective) observational study. 1.66 ALLEVYN LIFE Dressings vs 3.14 previous dressings (foams or gelling fibres); n=94; p<0.001). €11.46 ALLEVYN LIFE Dressings vs €27.75 previous dressings (foams or gelling fibres); n=94; p<0.001).
****n=38. Time saving based on previous dressing, estimated for 169 patients requiring ≥3 visits per week.
- AHRQ National Scorecard on Hospital-Acquired Conditions Updated Baseline Rates and Preliminary Results 2014–2017.
- Wassel CL, Delhougne G, Gayle JA, Dreyfus J, Larson B. Risk of readmissions, mortality, and hospital-acquired conditions across hospital-acquired pressure injury (HAPI) stages in a US National Hospital Discharge database. Int Wound J. 2020;1–11. https://doi.org/10.1111/iwj.13482.
- Atkinson, L. & Costa, B. (2024). “Pressure Injury Prevention with A Unique Multi-Layer Foam Dresing: A Systematic Review and Meta-Analysis of Randomized Controlled Trials” Poster presented at the 34th European Wound Management Association Annual Meeting, May 1-3, 2024, London, UK.
- Marché C, Creehan S, Gefen A. The frictional energy absorber effectiveness and its impact on the pressure ulcer prevention performance of multilayer dressings. Int Wound J. 2024 Apr;21(4):e14871. doi: 10.1111/iwj.14871. PMID: 38591160; PMCID: PMC11002638.
- Forni C, Searle R. A multilayer polyurethane foam dressing for pressure ulcer prevention in older hip fracture patients: an economic evaluation. J Wound Care. 2020 Feb 2;29(2):120-127. doi: 10.12968/jowc.2020.29.2.120. PMID: 32058851.
- Rossington A, Drysdale, K, and Winter R. Clinical performance and positive impact on patient wellbeing of ALLEVYN Life. Wounds UK. 2013;9(4):91-95.
- Smith+Nephew 2025. Wound Models testing over 4 Days – Horizontal, Moderate Flow, Viscous SWF and Wound Models Testing over 7 Days, Horizontal, Moderate Flow of ALLEVYN COMPLETE CARE Dressing. Internal report: CSD.AWM.25.009 V2.
- Smith+Nephew 2024. Comparison of Frictional Energy Absorber Effectiveness (FEAE) in two five-layer hydrocellular polyurethane foam dressings (HPFD) containing superabsorber and masking layers. Internal report: CSD.AWM.24.057 V2.
- Smith+Nephew 2025. Pressure redistribution testing of the ALLEVYN COMPLETE CARE dressing. Internal report: CSD.AWM.25.016.
- Austin M. Implementation of a Medical Device Related Pressure Injury Prevention Bundle: A Multidisciplinary Approach. Paper presented at: SAWC; 2019.
- Mcfee K. Implementation of medical-device related pressure injury prevention protocols: Prophylactic foam dressing packets for proned Covid-19 patients in the ICU. Paper presented at: NPIAP; 2022; Florida.
- Stephen-Haynes J, Bielby A, Searle R. The clinical performance of a silicone foam in an NHS community trust. Journal of Community Nursing. 2013;27(5):50 – 59.
- Smith+Nephew 2025. Conformability Testing of ALLEVYN COMPLETE CARE Dressing. Internal report: CSD.AWM.25.013.
- Smith+Nephew 2025. Extensibility Testing of ALLEVYN COMPLETE CARE Dressing. Internal report: CSD.AWM.25.012.
- Tiscar-González V, Menor-Rodríguez MJ, Rabadán-Sainz C, et al. Clinical and Economic Impact of Wound Care Using a Polyurethane Foam Multilayer Dressing. Adv Skin Wound Care. 2021;34(1):23-30.
- Atkinson, L., Allen, D. & Costa, B. (2024). “Decreased Weekly Dressing Changes with A Five Layer Foam Dressing In Mixed Etiology Wounds: A Systematic Literature Review And Meta-Analysis” Poster presented at the 34th European Wound Management Association Annual Meeting, May 1-3, 2024, London, UK.
- Costa B, Allen D. Results from an international survey: foam dressings with WTCI may enhance clinician confidence to extend wear times compared with other foam dressings. Poster presented at: the 33rd conference of the EWMA; May 3‒5, 2023; Milan, Italy.
- Hurd T, Allen D, Saunders C. Reduced weekly dressing changes with a five-layer foam dressing compared with other previously used dressings in wounds of mixed aetiology: results of a systematic literature review and meta‒analysis of clinical studies. Poster presented at: the 33rd conference of the EWMA; May 3‒5, 2023; Milan, Italy.
- S+N 2024. Comparison of Frictional Energy Absorber Effectiveness (FEAE) in two five-layer hydrocellular polyurethane foam dressings (HPFD) containing superabsorber and masking layers. CSD.AWM.24.057 V2.
- Gefen, A., Orlov, A., & Orlova, D. (2023). The Protective Efficacy of a New Soft Silicone Multi-Layer Dressing in Reducing the Heel Pressure Ulcer Risk. International Wound Journal. https://doi.org/10.1111/iwj.70764
Recall and Complaints
Customer will provide such support and assistance as S+N may reasonably request in the event of a general or limited voluntary or mandatory recall of the Product(s). Customer shall promptly report any complaint in respect of the Products to complaints@smith-nephew.com.