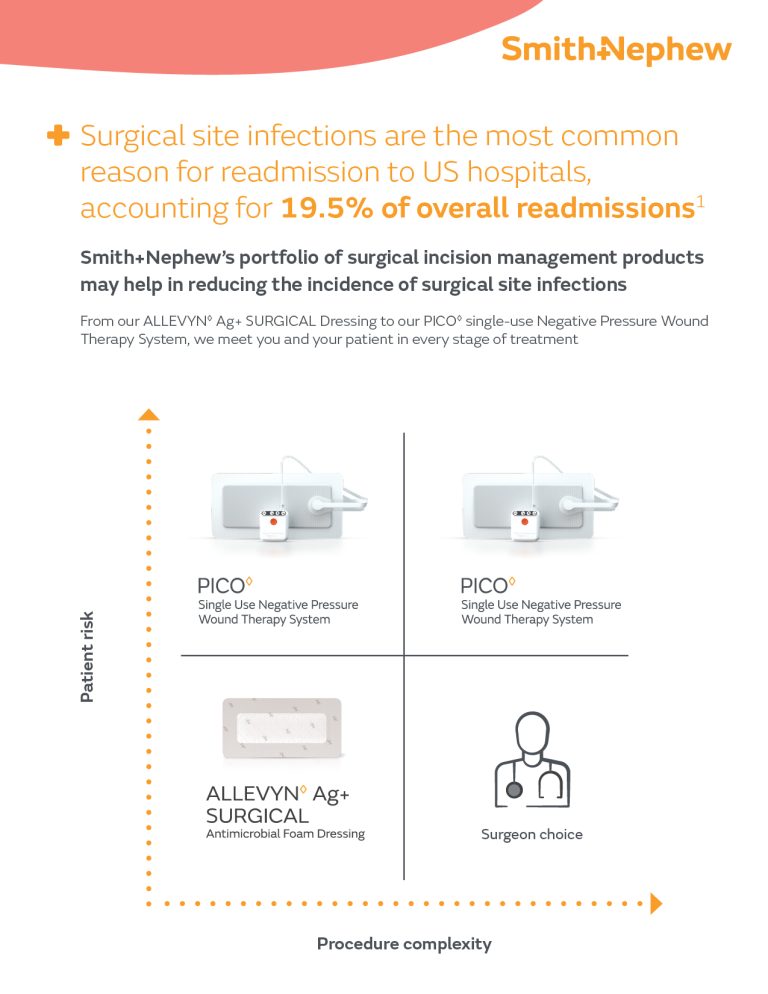
ALLEVYN◊ Ag+ SURGICAL
Antimicrobial Foam Dressing
Trusted to offer comfort and flexibility while reducing the bacterial bioburden in post-operative incision care.1-3
Why dressing selection matters
A patient’s experience of surgery extends beyond the operating room as they navigate their recovery and live with a surgical incision. If certain factors are disrupted or left unmanaged, it could lead to wound complications, discomfort and a potential delay in healing.
Consider how consistent, proactive use of an advanced foam dressing with sustained antimicrobial action1,2 could support clinical outcomes and patient experiences.

Discomfort
Impacting quality
of life
Bacterial bioburden
Increasing the chance of infection

Exudate leakage
Affecting patients’
self-esteem
Introducing ALLEVYN◊ Ag+ SURGICAL Dressings

ALLEVYN Ag+ SURGICAL mode of action
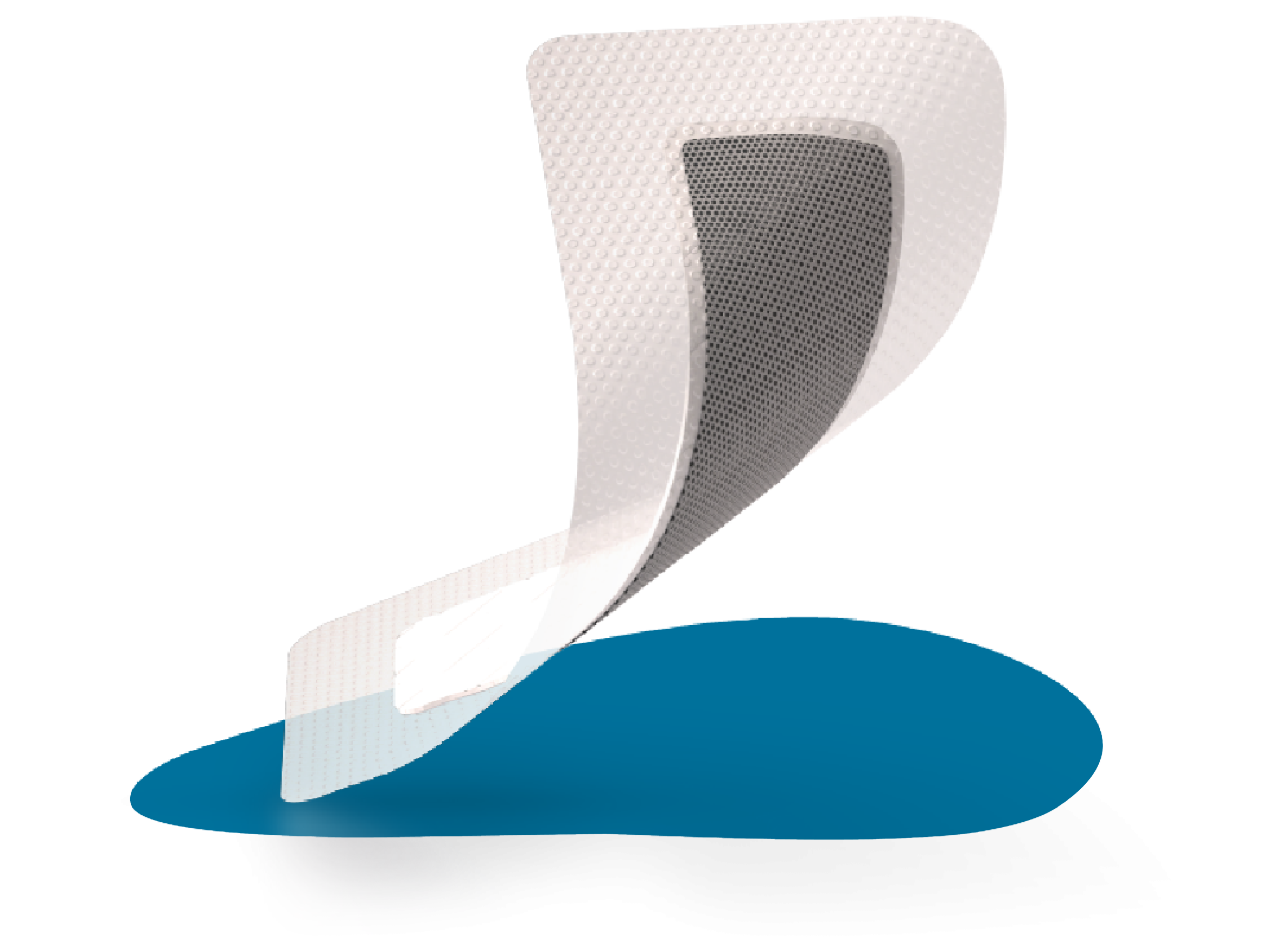
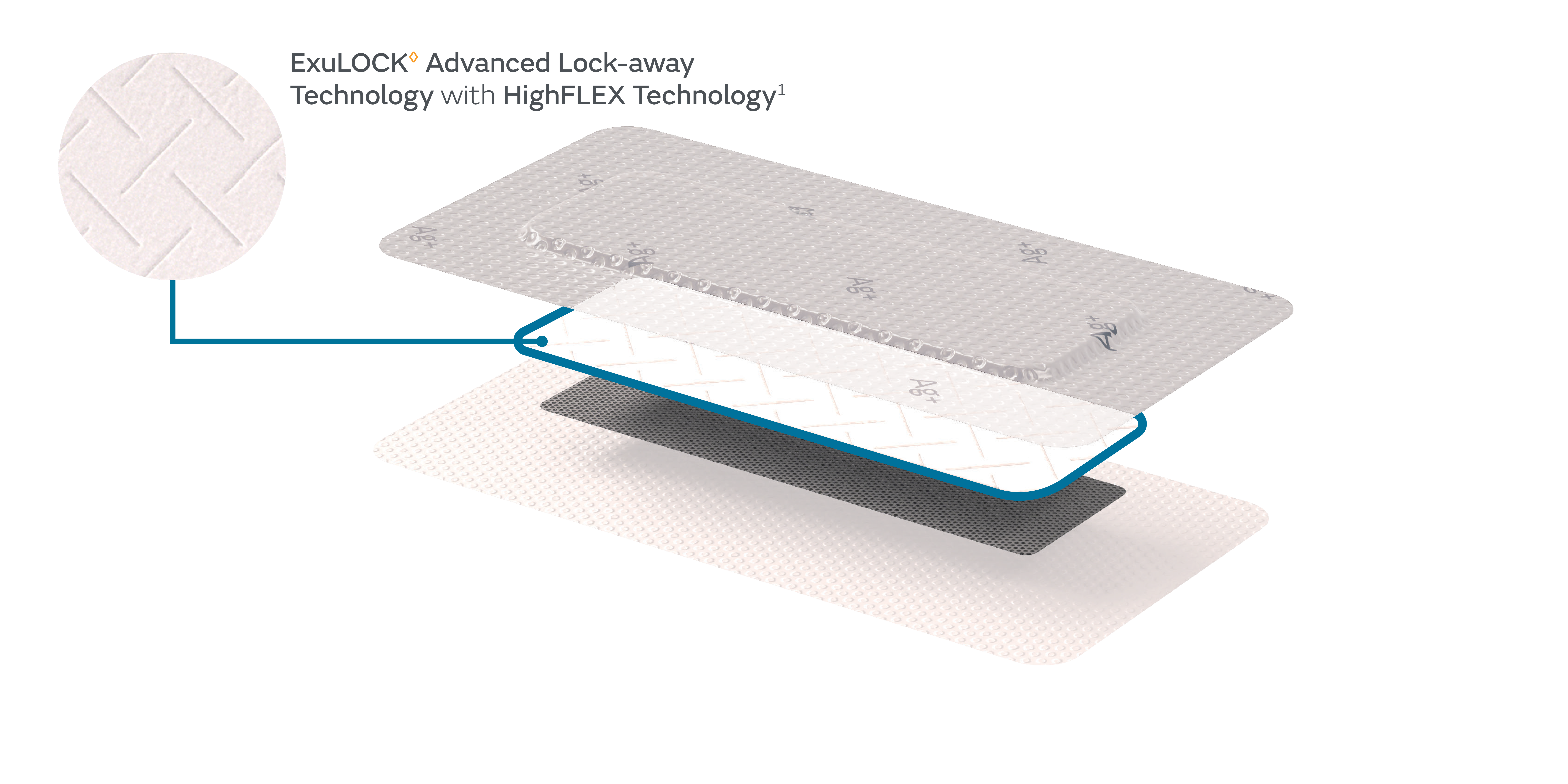
Inside the dressing
Examining the unique construction of the ALLEVYN Ag+ SURGICAL Dressing highlights why it can achieve such powerful results.
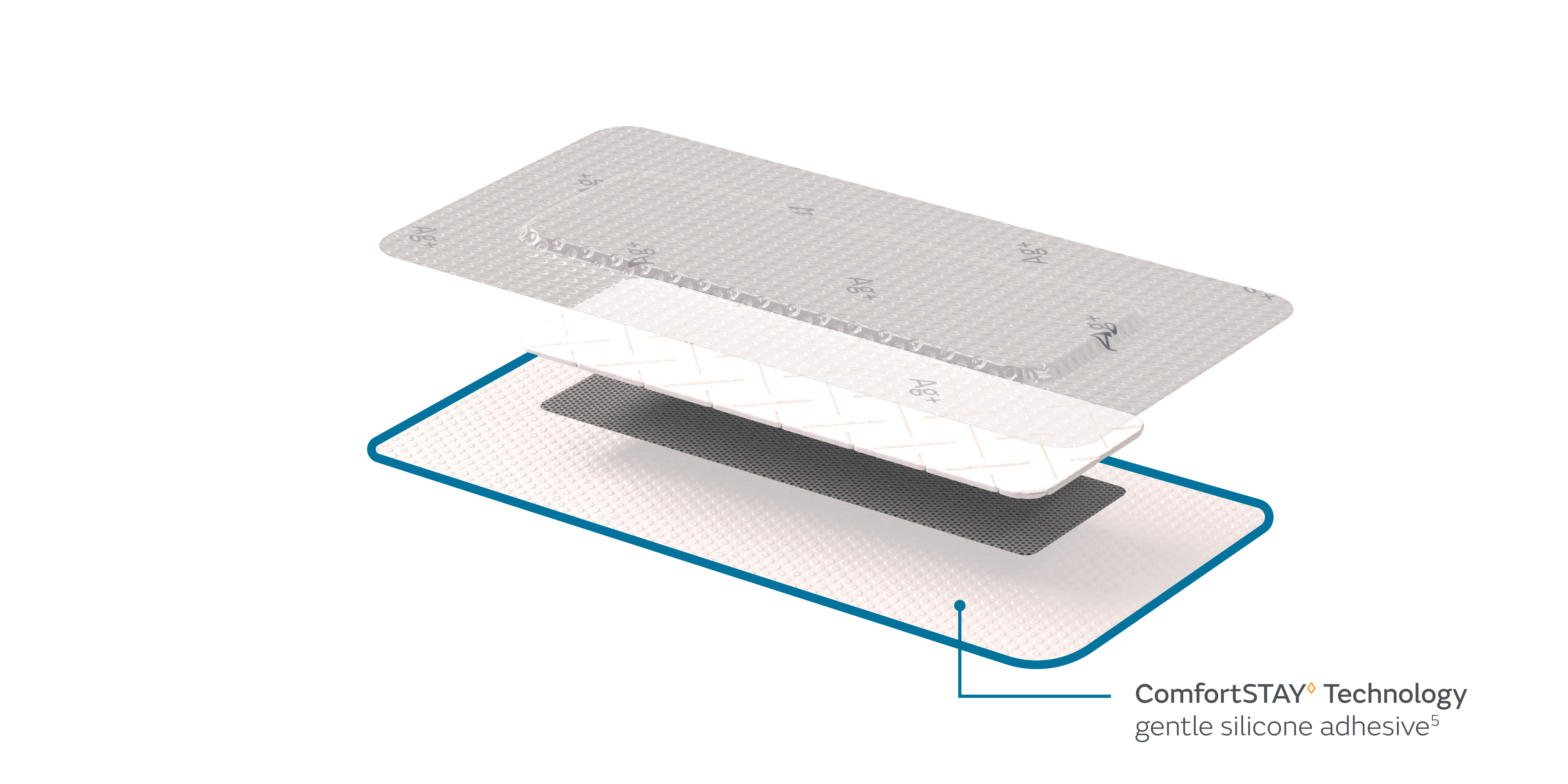
Wound contact layer
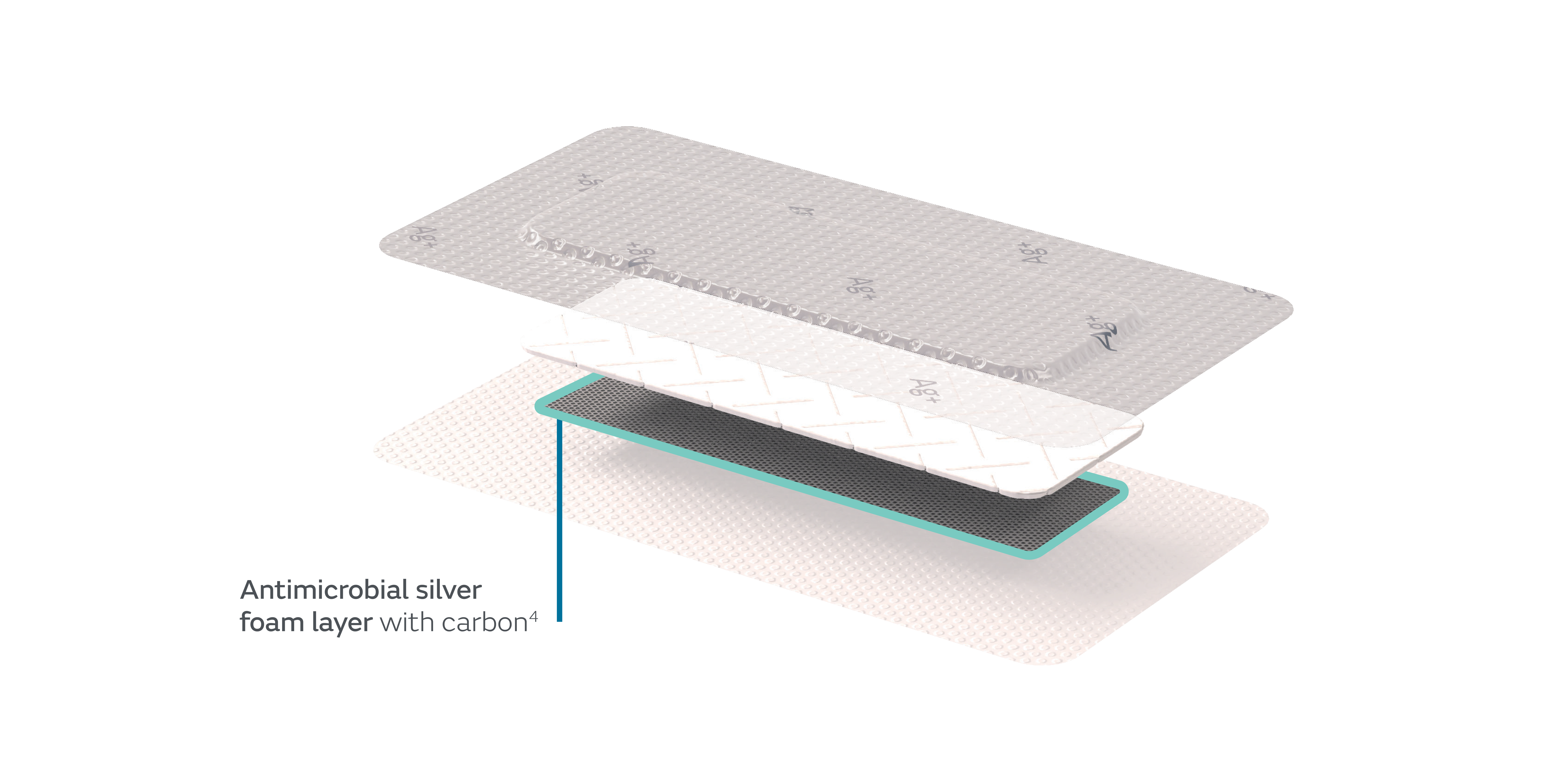
Silver foam layer with carbon
Superabsorbent core
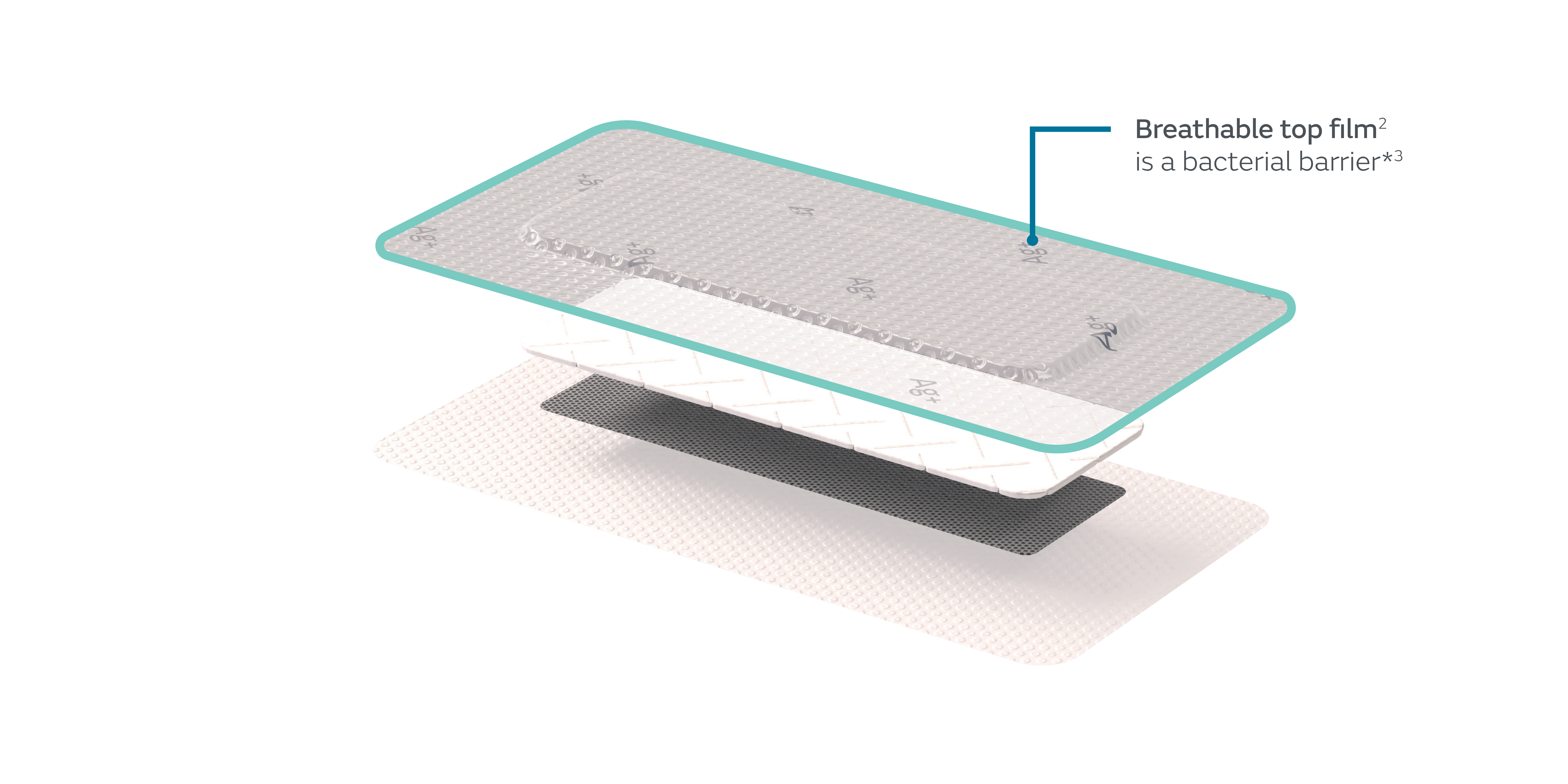
Top film layer
How ALLEVYN Ag+ SURGICAL Dressings work
For fragile skin
Non-sensitizing dressing composition is suitable for patients with fragile skin.16
Flexible and comfortable
Optimizes dressing retention and offers patient comfort during movement.3,15
Showerproof
Designed to support normal daily activities, allowing patients to shower.14
Antimicrobial efficacy
ALLEVYN Ag+ SURGICAL offers superior antimicrobial efficacy compared to other silver containing post-op dressings.*10-13
Minimize leakage
To help protect the skin, EXULOCK◊ Advanced Lock-in Technology helps minimize the leakage of wound fluid,4 using the entire dressing pad to help minimize the risk of periwound skin maceration.4-7
Clinical performance
Surgical teams need to have confidence in the dressings they use for their patients, and clinical testing has demonstrated why ALLEVYN Ag+ SURGICAL Dressings can be trusted to perform.
Superior antimicrobial efficacy
Superior to Mepilex™ Border Ag in reducing the bacterial bioburden in the dressing.**1
Up to 7 days’ wear time
Sustains antimicrobial efficacy for up to 7 days.*2
Effective against bacteria
Antimicrobial against common wound pathogens, providing a barrier against
small/motile species of bacteria and MRSA.*2,9
Support materials
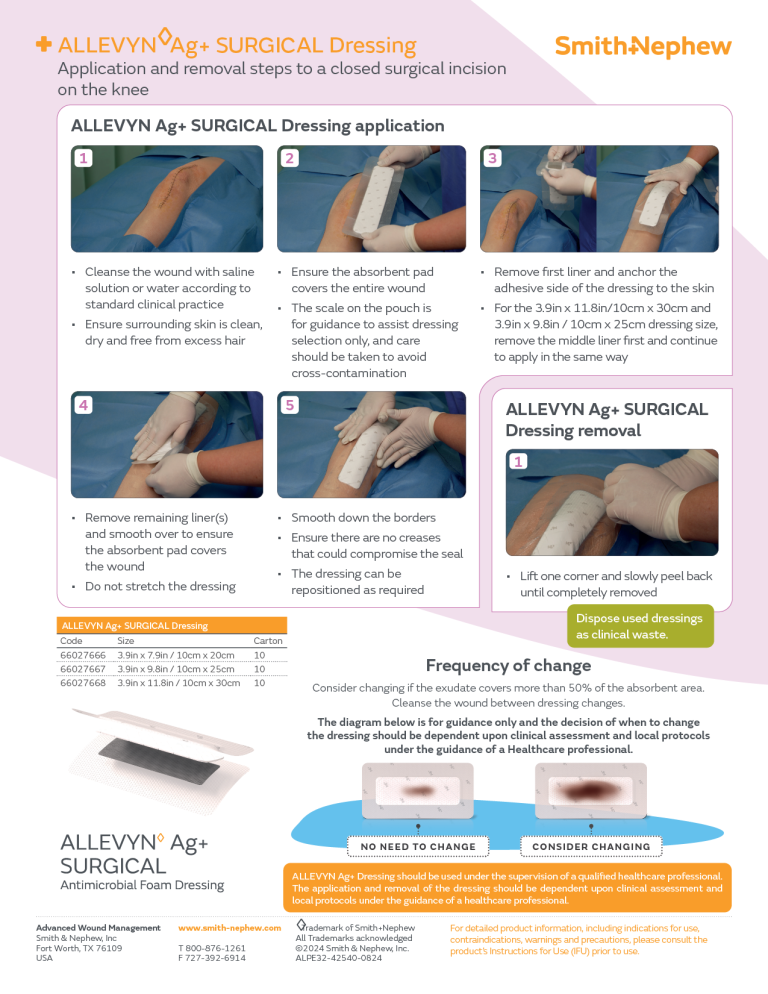
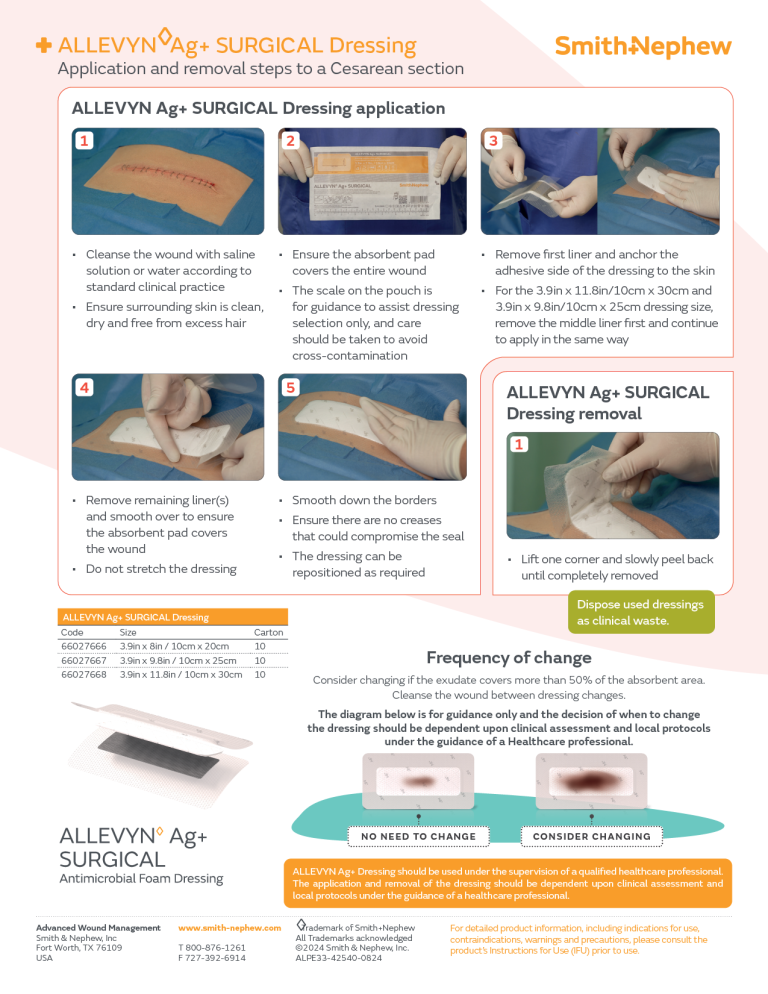
ALLEVYN Ag+ SURGICAL C-Section Application
ALLEVYN Ag+ SURGICAL Knee Application
Connect with us to request a demo
Share your email address for more information about how ALLEVYN Dressings can help your facility reduce costs and patient recovery times.

*As demonstrated in vitro.
**Tested in vivo using n=1 batch of each dressing. At day 1; p=0.006, day 3;
p=0.003, day 7; p=0.002.
Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Smith+Nephew representative or distributor if you have questions about the availability of Smith+Nephew products in your area. For detailed product information, including indications for use, contraindications, precautions and warnings, please consult the product’s applicable Instructions for Use (IFU) prior to use.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.014.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.019.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.025.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.024.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.033.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.092.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.026.
Smith+Nephew 2023. Internal Report. EA/AWM/RENASYS/009/v1.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.031.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.066.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.067.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.051.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.052.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.027.
Smith+Nephew 2024. Internal Report. CSD.AWM.24.030.
Smith+Nephew 2024. Internal Report. BE079.
Recall and Complaints
Customer will provide such support and assistance as S+N may reasonably request in the event of a general or limited voluntary or mandatory recall of the Product(s). Customer shall promptly report any complaint in respect of the Products to complaints@smith-nephew.com.